The Food and Drugs Authority (FDA) is warning the general public on hazardous eye drop brands that has caused the death and blindness of 3 and 8 persons respectively
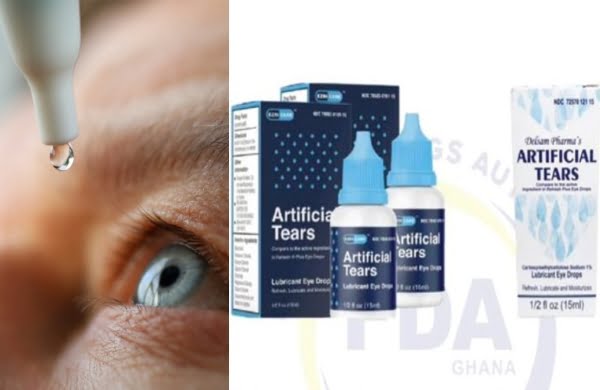
The two brands are reportedly on the Ghanaian market and still selling with names given as ‘Ezricare artificial tears’ and ‘Delsam Pharma’s.

fda 3
Both products are reportedly manufactured by Global Pharma – the FDA however mentioned that the products though recalled by the manufacturer voluntarily may still be on the Ghanaian market hence bringing it to consumers’ attention to avert further health problems and possible deaths.
The Food and Drugs Authority disclosed this to the public in a press release on its website adding that the Centre for Disease Control Prevention of the US has classified the incident as an outbreak.
It further revealed that the brands contains drug-resistant bacteria pseudomonas aeruginosa suspecting to be the cause of 3 deaths and causing the blindness in 8 individuals.
The products are not registered with the Ghana FDA but believe to be on the Ghanaian market, the FDA press release stated. It also advised the public that anyone in possession or may come across these brands must not use them.

1fda

fda
Ghanaians have been infuriated over the statement indicating that these drugs though not registered with the Food and Drugs Authority still entered the Ghanaian market. They remarked that the institutions are not up and doing and such harmful products shouldn’t reach the final consumer and cause deaths before identifying them.